When the C-STFT started its activities in 2005, no reference measurement system was available for free thyroid hormones (FT4, FT3). Therefore, the C-STFT started from scratch with defining the measurand, and proposing a reference measurement procedure (RMP) (5), (6).
Definition of the measurand
The measurand was defined as ‘‘Plasma/Serum - T4 (free); substance concentration (pmol/L)’’. According to the International Union of Pure and Applied Chemistry (IUPAC)/IFCC format, the component was ‘‘T4 that is not bound to proteins’’ (present in the water fraction of plasma or serum), the kind-of-quantity to measure the ‘‘amount-of-substance concentration’’ and the system ‘‘plasma or serum at physiological conditions (pH 7.40, temperature 37.0°C)’’. The preferred unit for expression of measurement results was pmol/L.
The conventional reference measurement procedure (cRMP)
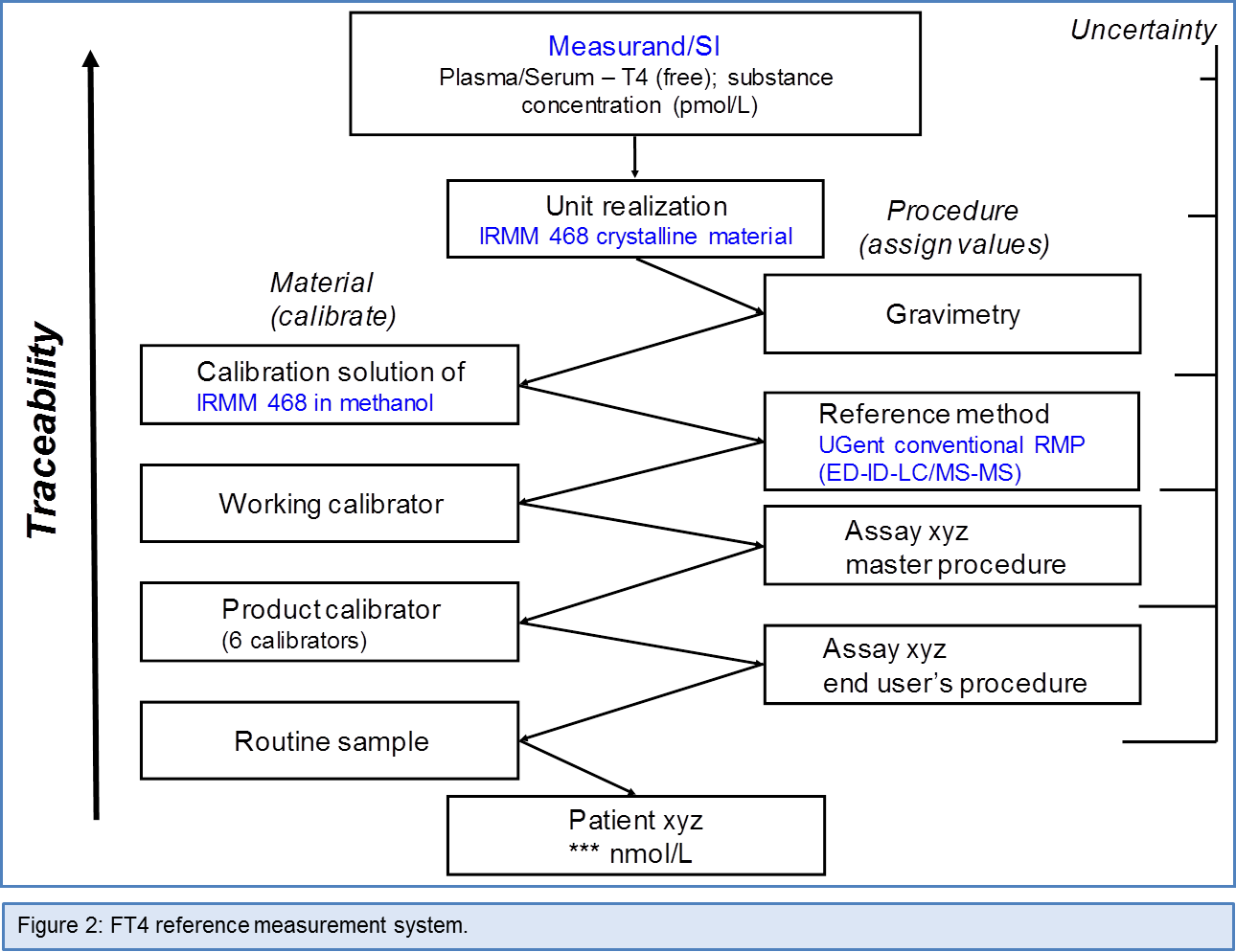
Ideally, metrological traceability of assays should be established with a trueness-based RMP as key element (see Figure 1). However, for free thyroid hormones, the development of a trueness-based measurement procedure is not obvious, because it has to include a physical step for separation of the free from bound fraction (by equilibrium dialysis (ED) or ultrafiltration (UF). Due to this separation step, it is by no means possible to unequivocally demonstrate that the T4 concentration in dialysate or ultrafiltrate is identical to the true free hormone concentration in the original sample, even if ED or UF is performed under ideal physiological and tonometric conditions. Therefore, the C-STFT decided to propose an international “conventional” RMP (further referred to as cRMP) for FT4 (note: FT4 was used as prototype, but the same applies to FT3). As explained in Ref. 6, they decided it should be based on ED and be combined with direct determination of the T4 concentration in the dialysate with a trueness-based RMP utilizing ID-liquid chromatography/ tandem MS (ID-LC/tandem MS) as measurement principle. The convention refers to the ED part of the cRMP, i.e. the C-STFT stated that it should be performed with strict adherence to a predefined procedure. This implied that according to this proposal the measurand was operationally defined as “T4 in the dialysate from ED of serum prepared under defined conditions”. The convention did not apply to the ID-LC/tandem MS part of the cRMP, provided it is calibrated with the certified T4 primary calibrator IRMM 468, already available in the total T4 reference measurement system (3). Again, the cRMP and the reference measurement services offered by UGent (= the reference laboratory of the C-STFT Chair) are listed in the JCTLM database (4). Figure 2 shows the FT4 reference measurement system.